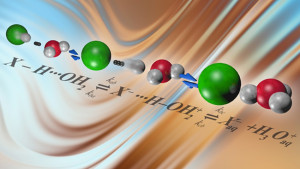
 Improving chemical reactions in fields from oil refining to battery life depends on understanding the chemistry of acids and bases. Researchers recently discovered that when a strong acid such as hydrochloric acid is mixed with water, the negatively charged anion and positively charged cation remain close and create an unexpected structure, suggesting a template for linking structure with function. The connection can help scientists develop a deeper understanding of the most basic concepts in chemistry and provide foundational information for use in battery applications.
Improving chemical reactions in fields from oil refining to battery life depends on understanding the chemistry of acids and bases. Researchers recently discovered that when a strong acid such as hydrochloric acid is mixed with water, the negatively charged anion and positively charged cation remain close and create an unexpected structure, suggesting a template for linking structure with function. The connection can help scientists develop a deeper understanding of the most basic concepts in chemistry and provide foundational information for use in battery applications.
Keeping the ions close
Research reveals that when a strong acid such mixes with water, the negatively charged anion and positively charged cation remain close and create an unexpected structure.