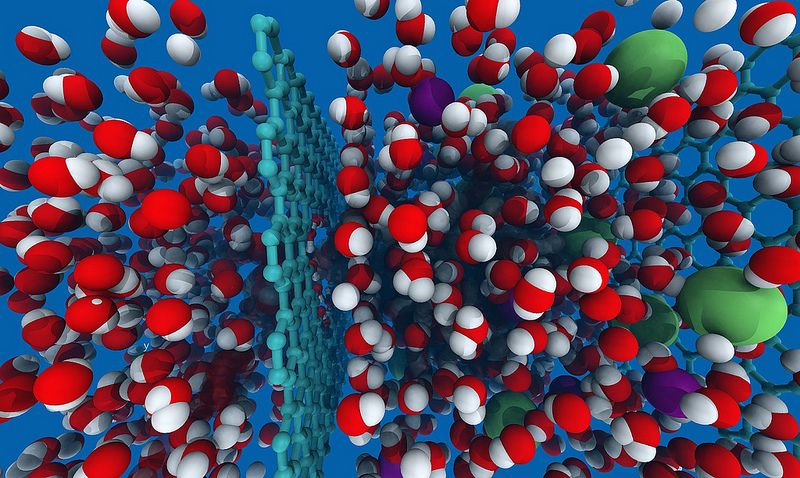
Guided by advanced molecular modeling on supercomputers, Massachusetts Institute of Technology scientists are investigating ways to turn atom-thick carbon layers into membranes for a new and improved desalination method in places with inadequate fresh water.
“Without any actual experimental demonstration, what our calculations tell us is that the performance of the graphene membrane for water desalination would be very high,” says Jeffrey Grossman, a materials scientist who is MIT’s Carl Richard Soderberg associate professor of power engineering and leader of the investigation.
Graphene, first described in 1962 and the focus of the 2010 Nobel Prize in physics, is a chicken-wire mesh of carbon atoms that provides the underpinnings for graphite, charcoal, carbon nanotubes and buckyballs. What has sparked the Grossman group’s interest is graphene’s phenomenal structural strength and chemical attributes that might make it ideal for filtering salt from seawater.
The goal is to drill just-the-right-width, billionth-of-a-meter nanopores into graphene’s normally impenetrable surface so pressurized water alone could get through without damaging the ultrathin structure. That might make it more efficient than the reverse osmosis (RO) process Grossman says now offers the best performance of all seawater desalination options.
Their computer sleuthing suggests that replacing reverse osmosis with graphene filtration could increase water flow rates by 100 to 1,000 times.
The problem is RO has comparatively high costs and energy use. Those faults mean that although seawater is widely available, “dramatically new technologies” are needed to make desalination a sustainable water supply option, Grossman and graduate student David Cohen-Tanugi reported earlier this year in the journal Nano Letters.
Climate continues to warm while freshwater resources become scarcer in some coastal communities, so “there are going to be many more places where I think desal is going to be a really important option,” Grossman says.
In osmosis, water spontaneously migrates through a semipermeable membrane toward the side on which a dissolved solute is more highly concentrated. The water builds up osmotic pressure as it moves.
In reverse osmosis, the favored desalination option, opposing pressure is introduced, switching the system’s direction. Water is forced to migrate to the membrane side where the solute concentration is lowest, separating it from dissolved salt ions.
A semipermeable membrane, which for desalination usually is made of polymers, is a thin film whose chemistry is tailored to pass water while excluding unwanted solutes.
Although water normally migrates via slow diffusion, RO requires high pressures to reverse this process and overcome osmotic pressure. Desalination rates can average about 250 million gallons a day at industrial RO plants, Cohen-Tanugi says, but at the cost of higher energy consumption.
In contrast, Grossman and Cohen-Tanugi think graphene could directly filter dissolved salts, further increasing water flow-through while potentially lowering energy demands. And its single-carbon-atom-thick sheets would be “the ultimate thin membrane,” their Nano Letters paper says – about 1,000 times skinnier than an RO semipermeable membrane.
Because graphene is the strongest material known, Grossman says, their studies suggest it could withstand perforation with tiny pores of just the right size to pass only water molecules. Meanwhile, salt ions – whose dimensions are naturally swollen by attached sheaths of boundary water molecules – would be nano-blocked.
The research also suggests the nanopores’ edges could be further modified to promote water flow. The MIT team’s calculations show the pore chemistry influences the flow rate as well as salt rejection.
The MIT researchers have deduced all this and more by tapping into two high-performance systems at the Department of Energy’s National Energy Research Scientific Computing Center (NERSC) at Lawrence Berkeley National Laboratory.
Accessing Hopper, a 153,408 processor-core Cray XE6 capable of 1.05 petaflops, plus Carver, an interconnected 3,200-core IBM iDataPlex system there, they used an advanced molecular dynamics simulation tool called LAMMPS to perform some exhaustive calculations, Grossman says.
“We went through millions of steps. These are simulations that were run on thousands of processors at a time, sometimes taking days or even weeks to run. That’s about the state of the art.”
Computer modeling is essential to modern-day chemistry and materials science because “it sits in between theory and experiment,” Grossman says. “It allows us to take theories and solve them for quasi-realistic systems that get you understanding those systems.
“Of course, what comes out of the computer is only as good as the model you put in. But I think we’re at a point now where we can include quite a bit of complexity in the simulation. We can do actually what an experiment would have a hard time doing, which is to peel away those levels of complexity one by one.
“As a materials scientist, what I stay up at night for is designing new materials for energy savings and water quality.” This investigation, which is proceeding to actual wet chemistry experiments, keeps both water and energy in focus. Despite RO’s being “really competitive compared to other approaches to desalination,” Grossman says, running at high pressures uses a lot of energy and drives up costs. “They’re still too high for RO to become the solution to the water problem.”
Their computer sleuthing to date suggests that replacing RO with graphene filtration could increase water flow rates by 100 to 1,000 times over diffusive RO membranes. Although high pressures still are a necessity, they could be lower using graphene than RO in other scenarios, according to the researchers’ calculations. Grossman cautions, though, that energy efficiencies could, “of course, never be boosted as much as the flow rates because of limitations imposed by the laws of thermodynamics.”
Actual experimentation, however, must first show whether the promising benchmarks that molecular modeling suggests would be achievable. Those include reliably creating enough nanopores of just the right diameter: 7 to 9 angstroms, or around 1 nanometer. It’s also unclear how to make such precise holes. Possibilities include molecular self-assembly, helium-ion bombardment and chemical etching.
Grossman joined MIT in 2009 and leads a research group of 12 graduate students, six postdoctoral researchers and a number of other scientists and undergraduates. While working as a postdoctoral fellow in physics at the University of California at Berkeley, he migrated toward materials science focusing on the global quest of creating new materials to address energy problems. He has since expanded to boosting clean water availability and other environmental challenges.