The Institute for Computational Biomedicine at Weill Cornell Medicine, Cornell University’s medical college in New York City, needs help from the world’s fastest supercomputer, says Harel Weinstein, the institute’s founding director.
“We work on the structure, function and biological mechanisms of extremely large molecular systems in living cells,” Weinstein says. “The systems we simulate on computers are made up of millions of atoms. In the computations, we follow the behavior of these molecules as their atoms change positions in time and space as they interact with one another and with medications under a variety of conditions.”
That means that his seven-person team needs to trace dancing atoms for nanoseconds and milliseconds — what Weinstein calls “extremely long times.” These movements produce trillions or more of data points that are collected by the Oak Ridge Leadership Computing Facility’s Frontier, the world’s first exascale computer. As Weinstein says, the team runs hundreds of iterations on the movements “to reflect the dynamics of the behavior of these molecules of life.”
Weinstein is the principal investigator for a 650,000 node hour INCITE project allocation on Frontier, which can perform more than a quintillion (1018) calculations per second. In particular, his group is tapping Frontier’s biomolecular modeling aptitude, which is enhanced by the artificial intelligence of its record-breaking machine learning hardware and algorithms. “Machine learning algorithms are activity discoverers,” Weinstein says.
In discovering how these systems behave, Weinstein’s group includes other Cornell researchers, among them assistant professor George Khelashvili, postdoctoral associate Ekaterina Kots, graduate students Shana Bergman and Hengyi Xie, scientific computing technology engineer Derek Shore and recent Ph.D. graduate Ambrose Plante, plus outside colleagues.
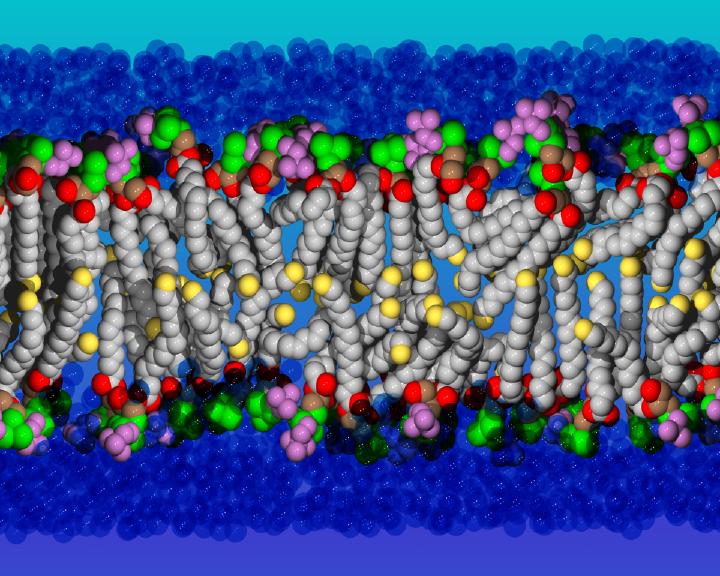
Their work produces nano-microscopic images of molecules with the use of various experimental techniques. One is X-ray crystallography, the storied method used to show the world what DNA looks like and continues to determine and display protein structures and other biological molecules. A newer technology is cryogenic electron microscopy, which scans the molecules as they dance in watery environments and freezes their motions at liquid-nitrogen temperatures so that the researchers get a sampling of some of the steps involved in those movements. To produce these images, Weinstein says, the collaborating researchers go to great pains to extract cellular proteins that can be purified for analysis. From all of this, Weinstein’s team can determine the three-dimensional structures and, thus, every atom’s coordinates at a particular time. “Once a computer has the coordinates, if you tell it to draw it, it draws it.”
‘We can publish snapshots and little videos that show everything that happens.’
These models are then fed into Frontier to start the simulations that capture the way the molecules move and change shape as they perform their functions in the cell. “Because we have the data, we can publish snapshots and little videos that show everything that happens,” he explains.
Weinstein’s group is especially interested in what happens to the molecules residing in cell membranes. These crucial entities are made up of enormous numbers of molecules called phospholipids that organize themselves in layers separating each cell from its neighbors. This is made possible by the structure of each phospholipid, which has an electrically charged phosphate head that likes the water that surrounds it. The other end has a long, fatty lipid tail that is repelled by water and points in the other direction. This is why phospholipids self-assemble in their watery environments into sealed pockets called bilayers.
Each cell also needs to communicate with its surroundings to obtain nutrients and participate in life-sustaining activities. Scientists have learned that this task is performed by million-atom proteins embedded in these lipid membrane systems.
Part of Weinstein’s Frontier project focuses on two key types of molecular machines that shuttle molecules from one side of the membrane to the other: lipid scramblase and transporter proteins. Their failure to perform is implicated in genetic disorders.
Scramblases are traffic cop-like proteins that put straying phospholipids back where they should be in cell membranes. If scramblases shirk this duty, the cell is no longer properly recognized by its environment, and disease ensues.
Transporters are like delivery vans that move around small and large molecules, as well as ions. One example is the transporter of dopamine, which is “an absolutely essential small molecule for the function of the brain,” Weinstein says. For instance, he notes that an absence of dopamine plays a role in Parkinson’s disease.
Both scramblases and transporters are “parts of our cells that help control what goes in and out, directing the movement and storage of energy,” Weinstein notes. And these molecular machines also “allow nutrients and vitamins to enter, and enable hormones made in the cell to be excreted.”
The sequencing of the human genome in 2002 made it “easy to find out that certain mutations are associated with certain diseases,” Weinstein says. “And these mutations are in almost all the genes we have.” Some are innocuous; some are not.
According to research by Weinstein’s group, genetic disfunction by just one type of scramblase can lead to disorders of muscles, bone, blood and brain. And problems in just one transporter are associated with primary microcephaly, intercranial hemorrhage and Alzheimer’s disease.
Drug abuse also can disrupt cell-membrane order. For example, cocaine “very specifically binds to the dopamine transporter and doesn’t let the dopamine back in,” Weinstein says. In previous work on the Oak Ridge Leadership Computing Facility’s Summit supercomputer, his group learned how to keep cocaine from binding with the serotonin transporter, which Weinstein says is nearly identical to the one for dopamine.
Much of the work by Weinstein’s group scrutinizes drug design and side effects. For example, these scientists are using Frontier to see if anticancer drugs could be made to hitchhike on a transporter protein called MFSD2A to breach our brain’s penetration barriers and treat tumors inside. In 2021, collaborating experimentalists at Columbia University used animal models to show that MFSD2A can convey the popular over-the-counter nutrient omega-3 fatty acid into the brain and eyes. “But nobody knew how it works,” Weinstein says.
Although Weinstein and his colleagues use supercomputer simulations to unravel such molecular mechanisms, that work must be confirmed in lab experiments to advance treatments. “Nobody is going to make a new drug or therapy because our computer predicted this mechanism,” he says. “They want verification.”