As solar-cell and semiconductor materials go, perovskite would seem to be an underdog when compared to such staples as silicon and gallium arsenide. The latter have dominated the chip and solar-energy worlds in recent decades. But might other, upstart materials like perovskite stage dramatic upsets over the reigning champions?
That’s what Carnegie Mellon University’s Noa Marom and the team she leads are attempting to predict, with help from an Innovative and Novel Computational Impact on Theory and Experiment (INCITE) award from the Department of Energy (DOE). The team has 320 million core hours on the Mira and Theta supercomputers at DOE’s Argonne National Laboratory (ANL).
“The idea is to find better materials for solar cells,” says Marom, assistant professor of materials science and engineering at CMU in Pittsburgh – both the light-grabbing compounds and their interfaces, the latter consisting of combinations of interacting substances. Organic-inorganic interfaces are one of three research thrusts in the new INCITE project at the ANL-based Argonne Leadership Computing Facility, an Office of Science national user facility.
“In an organic solar cell, you have active layers of organic materials” – ones that have carbon as an ingredient – “that harvest the sunlight,” she says. “Then these active layers interact with electrodes – metal electrodes, typically – to transport the current out of the solar cell.”
The active material in hybrid solar cells, meanwhile, consists of a combination of organic and inorganic components. That active material is itself a kind of interface.
“That means that we have to study how these materials work together, the properties of the interface, not just each individual material,” Marom explains. “We can point out promising materials, then have experimentalists try them.”
Marom and her associates focus their work on the atomistic scale. “For example, if your material is a molecular crystal, then we want to know the molecular structure of that crystal,” she says. “And if your active material is a layer of molecules on a metal electrode, we want to know how these molecules are arranged on top of the metal.”
Marom’s INCITE team employs an innovative approach that involves combining quantum mechanical simulations governed by the strange physics that reign at sub-atomic scale, optimization algorithms that compare multiple potential solutions to select the best one, and machine learning, or teaching computers to learn from experience, – all integrated into one work flow.
“In these simulations, we can’t solve the equations of quantum mechanics exactly for systems with many interacting electrons,” Marom says. “We have to use certain approximations in order to predict the properties of the systems.”
‘The simplest iterations of these materials are not long-term stable like our traditional semiconductors. This has to be fixed.’
One of those approximate methods is density functional theory, which allows the team to determine molecular structure. To calculate optical properties, the researchers use many-body perturbation theory. These two quantum mechanical methods let them cover the most interesting regions of chemical possibilities with remarkable accuracy, says Duke University’s Volker Blum, a member of the INCITE team.
The interface molecular systems are large and complex, thus the need for supercomputers.
“The calculations have to be very accurate,” Marom says, because many variable factors of many possible values govern simulations. That’s computationally challenging.
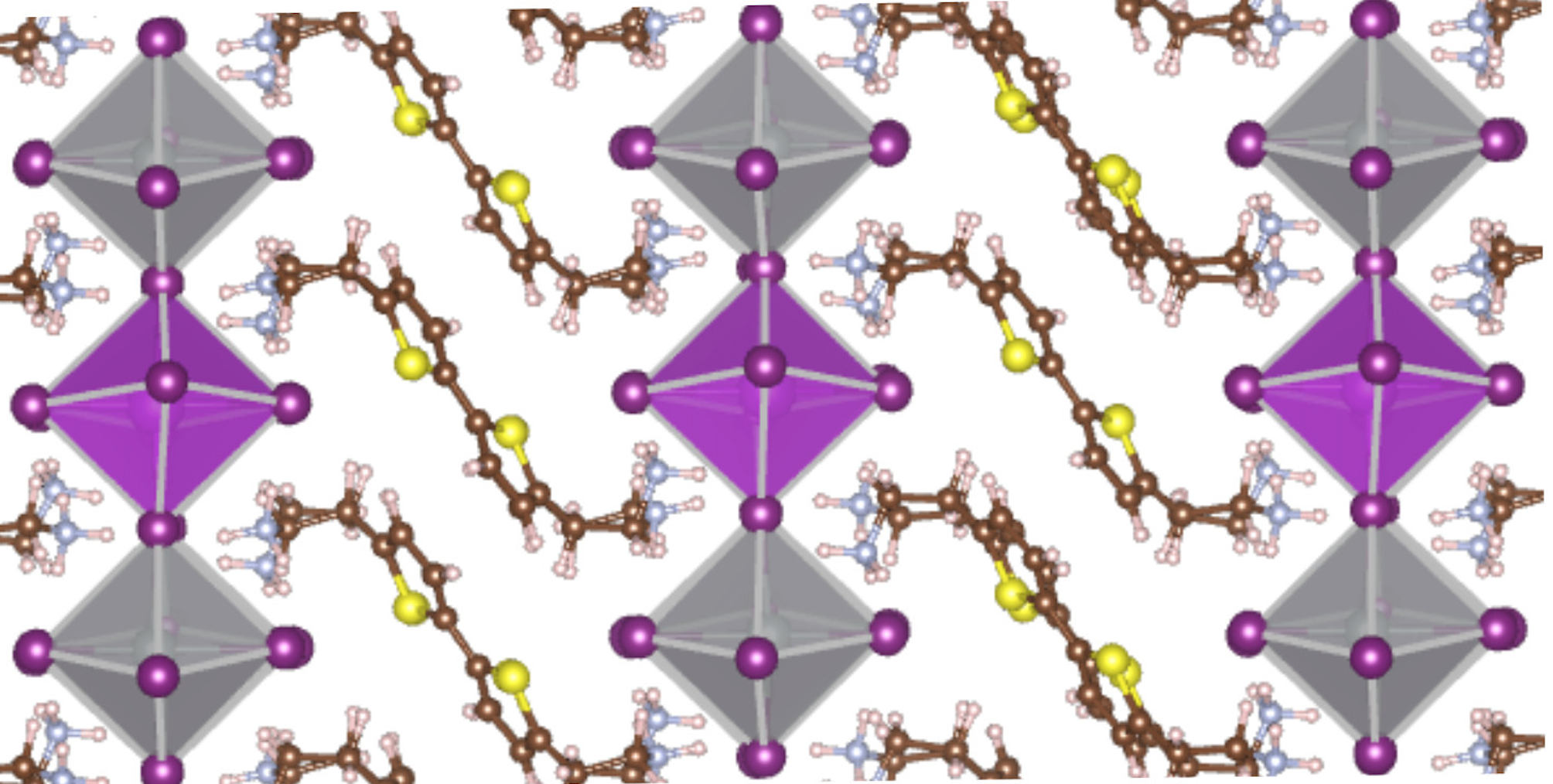
The other two research thrusts of the INCITE project are molecular crystals and organic-inorganic perovskites, minerals composed of calcium, titanium and oxide.
“In the molecular crystals thrust we have been able to identify a few new candidate materials for singlet fission solar cells, but no one has tried them out yet,” says Marom, referring to a class of organic devices that could dramatically raise solar-cell efficiency. They do so by enabling a light particle to excite two electrons for energy conversion instead of the usual one.
As for hybrid perovskites, Blum collaborates with experimentalist David Mitzi, who has made some of these materials. Mitzi, who works independently of the INCITE project, and Blum are both Duke University professors and affiliates of the Center for Hybrid Organic Inorganic Semiconductors for Energy, or CHOISE, a DOE Energy Frontier Research Center.
Perovskites offer a semiconductor material that may in principle provide similar electronic properties, or perhaps even better ones, at lower cost than a more expensively manufactured photovoltaic material such as gallium arsenide, says Blum, an associate professor of mechanical engineering and materials science at Duke.
Gallium arsenide and other materials with similar atomic structures have been used for a long time as semiconductors, Blum says, “that you can tune with very high accuracy and precision. Gallium arsenide is also used in all sorts of other semiconductor applications where silicon is not enough.”
One challenge the researchers must overcome is perovskite’s short life. “The simplest iterations of these materials are not long-term stable like our traditional semiconductors,” Blum says. A silicon solar cell, for example, can operate for 30 years, while prototype devices made of the newer organic-inorganic perovskites have lifetimes measured in months. “This has to be fixed.”
Blum stressed the importance of developing software that allows researchers to make real predictions for real materials. The process is often laborious, in Blum’s case involving input from more than a hundred software developers.
“Before we pushed the button, people spent years on the method,” Blum says. “The methods we use now were developed in the code between 2009 and 2012.” Published in 2015, those techniques serve as the core of his group’s current work.
In 1999 Mitzi, the Simon Family Professor of Engineering at Duke, synthesized a well-defined three-dimensional layered perovskite crystal structure, a complex organic-inorganic hybrid that Blum and his associates used to validate their methods.
“From there we could start using that atomic structure to predict electronic properties, which is what really costs computer time and where we really need a large computer,” Blum says. Thus the need for the INCITE award, which enables them to vary the material and make additional predictions.
“These are materials that exist in principle,” Blum points out, “although a crystal structure may not have been known. In other cases they’re entirely new materials. This is what we’re doing now.”
One such new material, a layered perovskite substitute for lead that uses a more environmentally friendly combination of silver and bismuth, has been synthesized by Mitzi and co-workers and computationally characterized by Blum’s group, as recently reported in the Journal of the American Chemical Society.
One of the challenges researchers face in sifting through complex materials is finding the structures that they want. This may require performing hundreds or even a thousand calculations for a single material before they even start predicting their electronic properties.
“In our case it’s the individual calculation that becomes large,” Blum says, “because we have materials that have relatively complex structure and internal interfaces. This system size automatically drives up the computational cost.