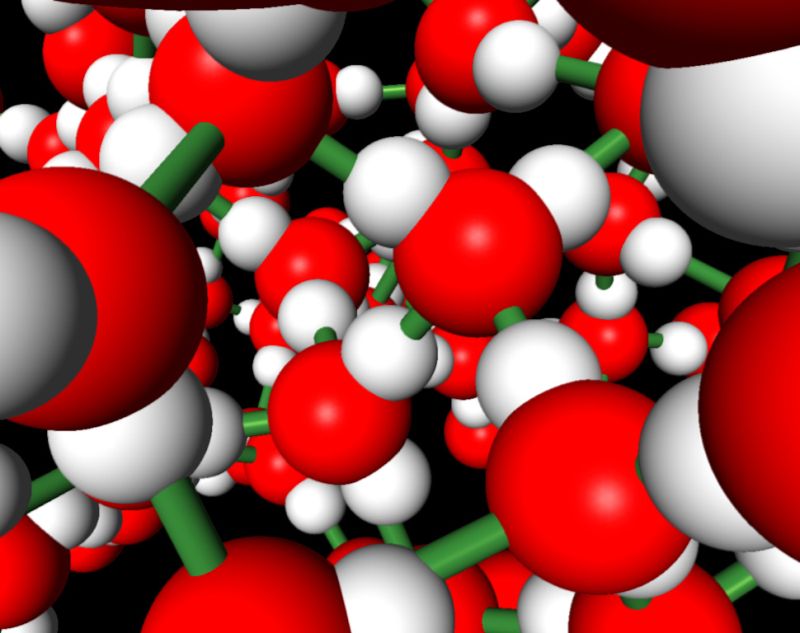
Water molecules seem simple, containing three atoms – two hydrogens and one oxygen. But this basic structure gets complicated as these molecules interact. Strong attractions between the molecules’ atoms lead to water’s high boiling point. Water molecules’ shape and organization in ice make it an unusual solid, with less density than the liquid state.
Because of these challenges and water’s importance for chemistry, biology, the environment and novel energy sources, researchers want a handle on how groups of water molecules behave around each other.
The Princeton University team of Robert A. DiStasio Jr., Biswajit Santra and Roberto Car has been studying water in depth on supercomputers. This year, they’re probing its microscopic structure with an ASCR Leadership Computing Challenge (ALCC) grant of time on Argonne National Laboratory supercomputers.
The idea is to fill in details about how water molecule groups interact, then make that information widely available. Armed with this knowledge, researchers may be able to build greener energy sources, such as aqueous batteries, and better understand a host of chemical reactions that occur in liquid water.
Battery technologies often depend on the use of organic solvents. As a renewable resource, water costs less and is nontoxic and nonflammable. Water-based battery materials could be non-acidic and have a lower environmental footprint, making disposal easier, DiStasio says.
If you zoom in on a single water molecule, understanding what’s going on is straightforward, DiStasio says. “Researchers can tell you anything you want to know about a single molecule, or even a few.” With a single molecule of water researchers can use highly accurate, quantum mechanical equations to model the details of both the nuclei and electrons of the component atoms.
These new calculations will be at least 100 times more computationally expensive than previous ones.
The trick comes when scientists plumb groups of molecules interacting, particularly in a condensed phase – liquid or solid. Water’s behavior in these systems is governed by a complex network of interactions between the hydrogen atoms on one molecule and the oxygen atom on another. These hydrogen bonds serve as a kind of adhesive that brings the molecules closer together. Those links also contribute to the highly disordered arrangement of molecules in liquid water, DiStasio says.
DiStasio and his colleagues are doing calculations on liquid water over a short time to find the location of every atom every 0.5 femtoseconds (one half of a quadrillionth of a second). As a result, they can get multiple snapshots of incredibly fast changes in water, such as the vibration of an oxygen-hydrogen bond in one molecule. But as researchers look at many molecules that resemble liquid water, they approximate, making the calculation manageable and less computationally demanding than it would be otherwise. Those approximations are where they face tradeoffs balancing accuracy with the size and expense of the calculations.
To get the most accurate calculation possible, DiStasio and his colleagues use two sets of equations, one that examines the behavior of the electrons and another for the nuclei.
The electron equations use density functional theory (DFT), a way to simplify calculations by relating the electron charge density to the energy of the system. “DFT represents the sweet spot between accuracy and cost,” DiStasio says.
However, standard DFT leads to an inaccurate picture of water and produces a structure that’s not fluidic enough, he says. Water molecules constantly vibrate, and in these simulations the molecular vibrations don’t match what occurs in experiments. Those results suggest that standard DFT isn’t giving an accurate picture of the hydrogen-bonding network in liquid water.
Therefore, DiStasio and his colleagues are using a DFT refinement called hybrid DFT. They can correct for interactions that are tweaking water’s vibrational profile and causing the oxygens of adjacent molecules to be spaced too widely. Using hybrid DFT comes with a price, though: Such calculations are at least 10 times more computationally expensive than those done previously.
The researchers also are looking at how non-bonded interactions affect groups of water molecules. Picture the electrons around the molecules as Nerf balls. As the balls interact, the foam can move and squish closer together. DiStasio and his colleagues have found ways to stabilize these more disordered but more realistic configurations and address broken hydrogen bonds without expanding the size or expense of the calculation.
Electrons have a much smaller mass than that of particles comprising nuclei at the center of the hydrogen and oxygen atoms, so researchers must further simplify the equations they use to model these parts of water molecules. For these calculations, they use classical physics based on Newton’s Second Law: Force equals mass times acceleration.
Although these simplified nuclei are computationally manageable, they’re not realistic. To address this problem, DiStasio and his colleagues have adjusted their calculations using equations famed physicist Richard Feynman developed that help them partially account for the detailed physics in the atomic nuclei. That adjustment adds another 10-fold cost to the calculation.
Overall, these new calculations will be at least 100 times more computationally expensive than previous ones, DiStasio says. “But it’s definitely one of the most accurate simulations of liquid water, and we’re trying to do it on the biggest sample that we can.”
The size of these calculations makes supercomputers essential. “The ALCC grant was instrumental in even trying to do this,” DiStasio says. From Princeton, he and his colleagues are developing algorithms to manage the data and make the most of Argonne’s parallel computing resources.
Water is just a starting point, DiStasio says. This calculation alone will require 40 million to 50 million processor hours on Mira at Argonne. They need those calculations before they can explore how other ions such as lithium, sodium, sulfate and fluoride behave in solution. The Argonne grant’s 250 million computing hours are probably enough for five or six simulations, DiStasio says.
With a better handle on water’s behavior and the effects of ions in water, researchers will have tools that not only could support the development of aqueous ionic batteries but also could lead to understanding properties such as acidity and alkalinity and ions in living organisms and in the environment.
After their yearlong study, the group’s calculations will be available online so all researchers can examine and build upon them. “The more you can understand about the basics of the system,” DiStasio says, “the more you can push the system to do what you would like it to do.”