Step on a Tesla Roadster’s accelerator, and “your head snaps back,” says Winfried Wilcke, electric-car enthusiast, pilot and Nanoscale Science and Technology Program director at IBM’s Almaden Research Center. With the near-instantaneous torque electric motors deliver, “it’s like a jet when you push the throttles forward – you are pushed hard against the seat,” he says from his office in the hills above San Jose, Calif.
Yet Wilcke hasn’t bought an electric car. A model like the Nissan Leaf won’t work for him. “I have a long commute, so it would run out of steam” he says of its 100-mile range. The Tesla boasts a 245-mile range, but it’s pricey and “is a little bit too small, although I’m still sorely tempted to get one.”
Wilcke exemplifies the conundrum manufacturers will have to overcome for Americans to adopt all-electric vehicles: The least expensive ones don’t go far enough to eliminate “range anxiety.” Models that go farther are too expensive or too small for most consumers.
These vehicles use lithium-ion batteries similar to those in laptop computers and cell phones. They hold far more energy for their weight than batteries of the past, but “they’re not going to enable us to have the kind of mobility that a tankful of gas gives us,” says Jack Wells, director of science at the National Center for Computational Science at Oak Ridge National Laboratory (ORNL). There’s a need, he says, “for something beyond lithium-ion. That’s opening up interesting science questions, many of which are fundamental.”
Wells leads a collaboration with IBM to help find the El Dorado of electric autos: a battery that’s light but packs enough power to take a car up to 500 miles without recharging. They’re armed with a grant of 25 million processor hours on Department of Energy (DOE) supercomputers from the INCITE (Innovative and Novel Computational Impact on Theory and Experiment) program, administered by the Office of Advanced Scientific Computing Research.
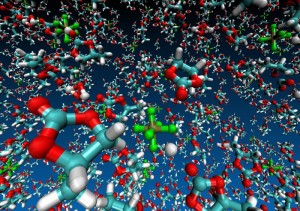
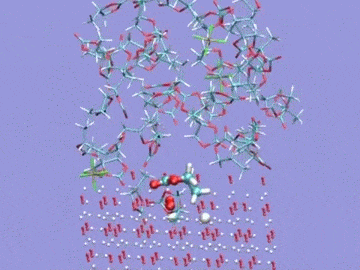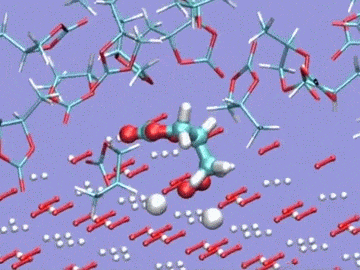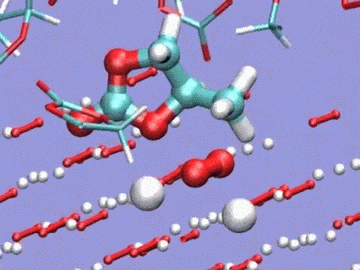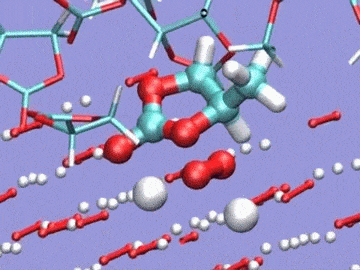
Argonne National Laboratory (ANL) researchers are running simulations on Intrepid, the lab’s IBM Blue Gene/P, to develop stable electrolytes for lithium-air batteries. In this visualization of an ab initio molecular dynamics calculation, the electrolyte is a silane-based polyether with a lithium hexafluorophosphate salt. The models are used to study lithium ion coordination and diffusion and the stability of the electrolyte molecule. Simulation by Kah Chun Lau, ANL.
The target: lithium-air batteries, with a projected energy density per unit of weight that’s five to 10 times that of lithium-ion technology. “Understanding the Ultimate Battery Chemistry: Rechargeable Lithium/Air” has 15 million processor hours on Intrepid, the IBM Blue Gene/P at Argonne National Laboratory (ANL) and 10 million on ORNL’s Jaguar, a Cray XT. That’s on top of a 24 million-hour allocation in 2010. Collaborators include researchers at both labs, at IBM, and at Vanderbilt University.
Lithium-air’s prospects as the ultimate battery have drawn plenty of attention, Wilcke says. In 2009, when IBM held a meeting to examine the issues, only a handful of research groups focused on lithium-air. “Now, I’ve lost track. It’s probably more than 50 organizations working on it worldwide.”
Translating formulae into reality isn’t easy, and the INCITE project already has challenged some of the conventional thinking.
First, a lesson in battery basics. A rechargeable lithium-ion battery is composed of a positively charged anode, a negatively charged cathode and an electrolyte between them. The electrolyte must conduct ions – atoms that have a charge because they’ve lost or gained electrons.
On paper, the equations are straightforward. In reality, the reactions are complex.
“When you connect a wire – an external conductor – from the anode to the cathode, electrons want to flow through that alternative path – an external circuit. That’s the whole point of a battery,” Wells says.
To create a current, positive lithium ions move from a carbon-based anode through the electrolyte to a metal-oxide cathode. Recharging the battery moves ions the other way, with electric current driving them into the anode for later release.
“The key thing is the ion doesn’t change its charge state” and still is positive, Wells says. “An old name still used for these kinds of batteries is a ‘rocking chair’ battery, because you’re basically moving an ion back and forth.”
Lithium ion batteries have become the favorite solution for applications that depend on high energy density – packing lots of charge into each unit of weight. “But you give up a lot of theoretical energy density when you don’t change the charge state of the ion,” Wells says, and lithium-ion cells still carry far less energy per unit of weight than gasoline.
Lithium-air batteries narrow that gap by taking some deep breaths. Oxygen from the ambient air provides the cathode; lithium metal forms the anode. When the battery discharges, the metal reacts with oxygen, releasing electrons and forming lithium peroxide (Li2O2 ) or, at higher currents, lithium oxide (Li2O). Recharging reverses the reaction, converting the oxides back to lithium metal and releasing oxygen.
That air-breathing quality is key to the new batteries’ lightness and high energy density. They aren’t burdened with a weighty metal cathode to hold ions when charged – although discharging will make them heavier as the reaction creates lithium oxides, Wells says.
On paper, the equations are straightforward. In reality, the reactions are complex. “One of the real challenges is we don’t understand the mechanisms,” Wells says. “This is what puts the problem into the realm of basic science.” Grasping these processes is critical, the INCITE team says, to conquering five major obstacles to successful lithium-air batteries:
Energy density far below the theoretical peak. Because electric motors are far more efficient than standard U.S. internal combustion engines, batteries need only store about 1,700 watt-hours per kilogram to match gasoline’s energy density, IBM-Almaden researchers say. That’s only 14.5 percent of lithium’s theoretical energy density, but basic research is needed to produce even that level.
Inefficient recharging. The voltage necessary to recharge lithium-air batteries is much higher than the discharge voltage. That’s because electrons aren’t just reacting with lithium oxides, Wells says. They’re also reacting with other battery chemicals, like the electrolyte.
Limited charge-discharge cycles. Laboratory lithium-air batteries can undergo only a handful of cycles before performance degrades, compared with hundreds for lithium-ion batteries.
Lithium’s explosive reaction with water. Either the battery material that admits air must also exclude moisture or the lithium anode must be coated to keep water out. Either could impede battery performance.
Limited power density. If energy density is like a tank storing power-producing fuel, power density is like an engine that actually delivers that power. Lithium-air batteries are like a small engine connected to a big tank: Lots of energy is available, but “it doesn’t like to give it off quickly,” Wilcke says. “Its power is low.”
To get at what’s happening, simulations focus on reactions at the interface between the cathode – such as a carbon-based material with tiny pores to admit oxygen – the electrolyte and ambient air. This three-phase boundary of solid, liquid and vapor is a classical engineering problem, Wells says, that’s further complicated by including the effects of prospective catalysts.
With its first INCITE allocation the researchers learned the lithium-air charge-discharge reaction was “a far wilder story than everybody had expected,” Wilcke says.
The first experiments tested the most likely electrolyte candidates: ones already used in lithium-ion batteries, such as propylene carbonate (PC). “A few papers were published claiming that one can indeed see cycling behavior, meaning you put power in, take power out, power in, power out,” Wilcke says. “That news was all completely wrong.”
Running on Intrepid, Teodoro Laino and Alessandro Curioni of IBM’s Zurich Research Laboratory simulated the interaction of lithium peroxide with oxygen in the presence of different electrolytes. In short order, Wilcke says, the calculations showed that the PC electrolyte is consumed during discharge.
At the same time, Almaden scientists were getting similar results in their experiments. They set up a lithium-air battery with a PC electrolyte and analyzed the gases it produced during discharge and recharge. Discharge produced no significant gas, as expected. Recharging should have produced oxygen, but instead generated mostly carbon dioxide. Further tests using carbon isotopes (versions of the element with extra neutrons) to trace the gas source found PC generated most of the carbon.
“The community is now really moving in a different direction, vis-a-vis the choice of electrolytes, and computational simulations gave early insight into that,” Wells says.
IBM and Argonne researchers are among those trying new electrolytes. The IBM Almaden group is testing candidates that the Zurich team’s simulations predict will be stable. Argonne researchers are studying other electrolytes their simulations predict, says Larry Curtiss, a distinguished fellow and group leader in the Materials Science Division.
“We’re doing some calculations to try to find how to change the (electrolyte’s) composition or the salts mixed with it to make it even more stable. It’s a computational materials design effort that’s making suggestions for experiments. We’re also using computations of lithium oxide nanoparticles to test electrolyte stability.”
That tight loop between computation and experiment is accelerating the pace toward a practical lithium-air battery, Wilcke says. “That’s how it should be and how it needs to be. Computational materials science is not yet far enough along on its own, and a loop is what you always want.”
Yet, Wilcke adds, computers like Intrepid and Jaguar, capable of more than a quadrillion calculations per second, have the potential to predict material behavior. And exascale computers, a thousand times faster than today’s, “will really have a big impact on materials science.”
Other lithium-air battery obstacles the INCITE project studies include the buildup of lithium oxide solids during discharge. “If you produce an insoluble material it can clog your cathode,” blocking air needed for the reaction, Wells says, “or precipitate out, just piling up somewhere.” Lithium peroxide also is an insulator, so the buildup can prematurely stop the discharge reaction.
“There will be basic science aspects of those problems we’ll be working on, but ultimately there will be engineering solutions,” Wells says.
Wilcke hopes to build a large-scale prototype in two years, but that’s still a long way from a commercial lithium-air battery.
“I’ll put it this way: It’s between 2020 and never,” Wilcke says with a laugh. “But I would bet it’s closer to real – to the first of the two.”